Operationalize Cell & Gene Therapies

As you aim to bring your advanced therapies to patients in need, partnering to overcome the associated regulatory, manufacturing, and logistical complexities is essential to avoid unnecessary costs and delays.
Leading the Way in Cell and Gene Therapy Commercialization
There is no question about the incredible clinical impact cell and gene therapies (CGTs) can have on the lives of patients. However, this impact is the result of innovation that does not equate to a simple, straightforward commercialization process. The commercialization and management requirements of these treatments are just as complex as their innovative science.
Commercialization of CGTs is significantly more challenging compared to traditional biopharma products. Their inherent complexity impacts distribution, access, affordability and patient support.
CGTs hold great life-saving and therapeutic advancement potential, but their complexity requires a careful approach–starting with engaging and educating patient communities earlier.
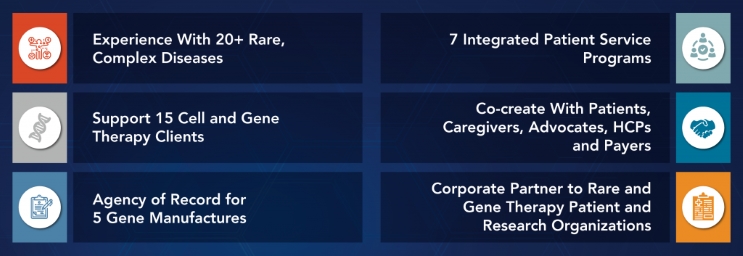
Leveraging CGT Expertise from Product Development Through Launch
As the industry focuses more on specialty therapies, it is imperative that manufacturers move to a patient-centered care model that delivers value enabled by innovative technologies and measured by actionable data. EVERSANA incorporates the patient perspective throughout the lifecycle of CGTs. We partner with innovators to think differently about their commercialization strategies, channel distribution, patient support and capability investments for market readiness.
We leverage our CGT expertise to bring together strategy, patient recruitment for clinical trials, HCPs and patient engagement across the patient journey, and warehousing and logistics to support the complexities associated with cell and gene therapies.
We incorporate the patient perspective throughout the lifecycle of cell and gene therapies.
Pre-Clinical
Infuse the patient voice into trial programs
- Meaningful clinical trial endpoints
- Clinical trial support and resources
- Form ongoing partnerships
Clinical/Pre-Launch
Provide education and resources to support trials and community
- Disease education
- FDA Advisory Committee meetings
- Patient advocacy group partnerships
Commercial
Identify how patient needs intersect with brand objectives
- Insight-driven marketing materials
- Co-created resources and support
- Patient programs, such as approved Risk Evaluation and Mitigation Strategy (REMS) programs
Click here to learn more about our Cell & Gene Therapy Solutions.
