Analytical Validation, Computer System Validation, And Bioinformatics Consulting

Accelerate and streamline your validation process with technical project management of your lab's analytical validation. Our analytical validation consulting services provide technical project management of your lab’s AV, potentially reducing your overall time to launch by 75%.
Keep launching new molecular tests. We've got your back.
End-to-end solution for your lab's analytical validation project
Testing laboratories are under constant pressure to stay compliant with standards while expanding the number of molecular tests offered. Validating a workflow is one of the most critical steps prior to launching a new test successfully. Yet, many labs inadequately plan and execute their analytical validation (AV) process. An inefficient AV can potentially turn a 10-week validation into a 40-week or more process, resulting in substantial delays to the lab’s ability to process valuable samples and significantly impacting the lab's business.
With over 400 successful AV engagements to date, our experienced AV project managers and global compliance service specialists help ensure successful AV outcomes.
- Save Time—Get personalized consultation to help accelerate your launch up to 75% faster than the average AV, enabling laboratories to get into routine testing faster and accelerate the return on investment.
- Cut Costs—Help reduce costs and provide transparency of your end-to-end investment with up to 50% less financial impact than the average AV.
- Maintain Compliance—Receive proper documentation templates that facilitate your lab compliance. Our experienced consulting team will assist in designing a proper sample run strategy to establish test performance metrics.
Enabling you to reduce your assay validation timeline and overall investment
See an overview of the steps of the AV process and how our AV consulting services can help reduce your timeline for launching new molecular tests.
Analytical validation workflow completed up to 75% faster with AV consulting service
The AV consulting services can reduce your overall investment by up to 50% because of the time savings the service provides. Labs that perform AV themselves could pay more in materials and labor, potentially incurring months of loss in revenue opportunity due to delays in sample processing.
Unsure about the AV process or need a wet lab assay bridging solution?
Consider starting with a pre-validation evaluation. Our analytical performance verification (APV) solution service helps save you time and costs by assisting you in determining whether your workflow is ready to move on to a complete analytical validation or can provide verification of wet lab changes to your assay workflow.
Guided by our global compliance services specialist (GCSS), the APV service enables you to evaluate your workflow on a controlled scale, and helps you improve your chances for success.
APV control kit
- Final analysis report
- Evaluation consultation
- Readiness checklist
Regional service
Our Analytical Validation consulting service is flexible and adapts to regional validation requirements.
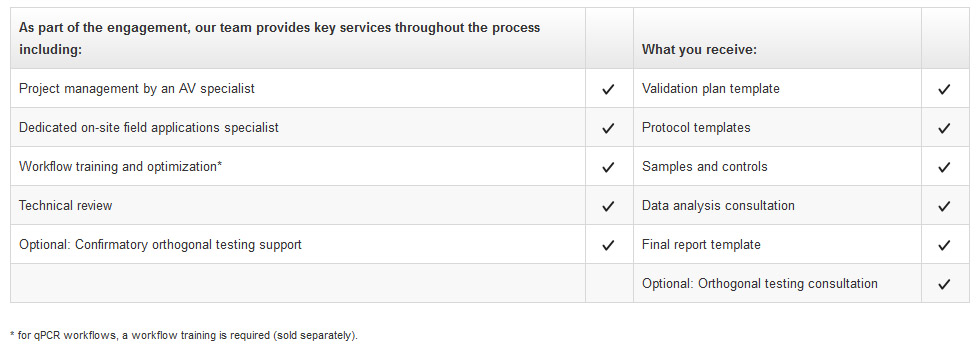
The Analytical Validation Regional (AVR) service is designed to follow regional requirements globally. The AVR is guided by a global compliance service specialist (GCSS). This takes you through an analytical validation process tailored to fit your global region. The AVR service includes:
- Project management by an AV specialist
- Samples and controls
- Data analysis consultation
- Template documentation
