Business Process Outsourcing
PRODUCTS
-
AGC Biologics develops and manufactures lenti viral vectors and retro viral vectors. Our ready-to-use platform capabilities are built on cell factories (up to 48L) and bioreactors (up to 200L) using adherent processes, designed entirely in-house. Our quality systems, GMP manufacturing scale and regulatory qualification allow us to meet both clinical and commercial demand. Moreover, our scale down capabilities provide flexible and cost-effective solutions for process development and pre-clinical studies. Since we perform more than 100 analytical tests in-house, AGC Biologics reduces overall turnaround time. With experience manufacturing three commercial products, we are the only Cell & Gene Therapy (C>) CDMO that has brought its own product to the market (Zalmoxis®).
-
Streamline data, maximize efficiencies, tighten up costs, and reach end goals faster using Medrio’s powerful and intuitive EDC tool.
-
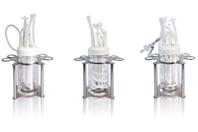
Optimized performance and fast bioreactor setup
Single-use bioreactors provide higher throughput thanks to easy setup and operation. Plus minimal operator handling reduces the ever-present risk of contamination. The Applikon AppliFlex ST has been designed to make your life in the lab easy.
-
What is included in Forge's DMF?
DMFs come in all shapes and sizes, but Forge's DMF includes the following components:
- Information on our critical raw materials, including our Ignition Cells™ and pEMBR™ adenovirus helper plasmid
- An overview of our platform AAV manufacturing process
- Process controls
- Facility layout and engineering information
- And more!
-
Second, only to Oncology, CNS, and Neurology clinical trials are incredibly complex and complicated to navigate.
WHITE PAPERS AND CASE STUDIES
-
Biological Indicators And The European Pharmacopoeia
Here, we review the information concerning biological indicators in European Pharmacopoeia General Chapter 5.1.2, which is more detailed and covers a wider scope than the previous version.
-
New Life For Pumping Stations
Read about ambitious rehab projects that bring pump stations up to date and keep wastewater flowing.
-
The Essential Role Of Adjudication Technology In Clinical Trials
With adjudication, learn how teams can enhance the quality and integrity of their research, paving the way for impactful and reliable outcomes in clinical research.
-
Searching For A CDMO To Support Affordable Conjugate Vaccines
Learn how a CDMO that prioritizes custom, flexible partnership over a transactional relationship is crucial for long-term programs and for tailoring services to meet the needs of small-volume clients.
-
Patient Diversity: Identifying Challenges, Opportunities, And Best Practices
By listening to the voice of patient diversity and implementing FDA guidance measures, learn how stakeholders can improve trust, engagement, data reliability, patient outcomes, and promote public health.
-
A Site Network’s Experience With eSource Before And During COVID
Discover how the world's leading vaccine network adopted eSource during COVID-19 despite many obstacles that got in their way.
-
Unlocking The Potential Of Decentralized Clinical Trials
Explore the current DCT landscape and its evolution, as well as the key advantages of incorporating decentralized attributes and how barriers to their inclusion can be overcome.
-
How To Save 9k Hours And Cut Deviations By 27%
This pharma company transitioned off paper, slashing their deviation count and saving thousands in onsite hours. Learn how they were able to streamline business operations and free up resources.
-
Electronic Batch Reporting
Learn how a CDMO automatically created a comprehensive batch report model to drive their reporting and speed root cause identification of quality failures with an Industrial DataOps software solution.
-
FACS Bulk Enrichment In Combination With Cell Cloning Systems Delivers Significant Savings For A CDMO
Learn how the Cell Line Development (CLD) department at Celonic Ag was able to optimize their workflow with new technology geared towards shortening time lines and lightening workload.
-
Understanding CDMO Selections: Getting It Right
Learn the importance of identifying CDMO key decision-makers and exploring how they reach determinations that reshape imprecise product-based marketing into messaging that addresses specific customer concerns.
-
Benefits Of Implementing EDC & Medical Coding With RTSM
Discover what happened when the Fountayn Platform, including RTSM, was used to support a client’s two-year, Phase III study seeking a cure for multiple sclerosis.
NEWS
-
STT Logistics Group Announces The Launch Of Their Corporate Account Service4/3/2023
Miami-based transportation, Logistics, Supply Chain, and Storage solutions provider, STT Logistics Group, announces the launch of their Corporate Account Service. The newly launched service aims to provide larger companies with comprehensive logistics and transportation services.
-
Meet 'Coscientist,' Your AI Lab Partner12/20/2023
In less time than it will take you to read this article, an artificial intelligence-driven system was able to autonomously learn about certain Nobel Prize-winning chemical reactions and design a successful laboratory procedure to make them. The AI did all that in just a few minutes — and nailed it on the first try.
-
An Unexpected Way To Upcycle: Plastic Waste Transforms Into Soap8/10/2023
A team led by Virginia Tech researchers has developed a new method for upcycling plastics into high-value chemicals known as surfactants, which are used to create soap, detergent, and more.
-
It's So Metal: Scientists Confirm Nickel Plays A Key Role In An Ancient Chemical Reaction8/18/2023
SLAC scientists showed that a carbon-metal compound with a perfectly placed nickel atom plays a key role in converting carbon dioxide into components for food and energy
-
Disarming Pathogens: New Drug Candidates To Combat Chronic Infections1/23/2023
The pathogen Pseudomonas aeruginosa is the cause of a large number of serious infections and places a particular burden on immunocompromised patients. The increasing spread of antimicrobial resistance makes it even more difficult to combat the dreaded hospital pathogen.