Forms Processing
PRODUCTS
-
Ascendia specializes in the development of market suitable formulations for poorly water soluble drug substances. Based on the pre-formulation data package and the delivery requirements for the product, we recommend a comprehensive formulation development strategy. We understand that each formulation is unique and must address the specific challenges associated with poor solubility, inadequate bioavailability, and/or unacceptable physical/chemical stability. We also understand the urgency companies have for progressing their pipeline, and our approach maximizes the probability of success by exploring multiple formulation options in parallel if necessary.
-
inSeption’s Clinical Operations and Project Management subject matter experts are integrated into the client’s project team, thus ensuring a more streamlined approach to providing the required support and/or recommending ways to achieve new efficiencies.
-
When AST came to the table on the design for our new fill-finish isolator, one of the first areas AST engineers wanted to address was the usability and accessibility of the isolator and corresponding operations. With our customers’ point of view in mind, we wanted to address specific points of friction routinely encountered by operators and closely examine whether those friction points were necessary.
Does an isolator need to be ergonomically unfriendly to clean?
Should simple mechanisms like isolator doors be challenging to engage? (As much as one can appreciate exercise, no one wants to do “arm day” in cleanroom coveralls).
Does routine maintenance have to be time-consuming and laborious?
Many of these factors are accepted as par for the course in aseptic fill-finish manufacturing. Our question was, why?
-
Zelta’s EDC has been used in more than 4,000 studies from startup to submission, across all phases, including over 500 phase 3c trials and 23 therapeutic areas.
-
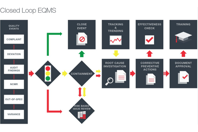
Managing documents, training, and business processes are nightmares shared by all pharmaceutical and biotech companies and MasterControl provides pharmaceutical quality management software systems to take them out from that nightmare. MasterControl's QMS software exchanges the nightmares for a dream of a solution that enables pharma companies get their products to market faster and improve compliance at the same time. MasterControl pharma QMS software has been specifically designed to help manage all documents and automate regulatory-related processes and training in a single platform.
WHITE PAPERS AND CASE STUDIES
-
AI Best Practices For Business Decision Makers And Practitioners
With companies in every industry embracing AI, it has never been more important for technical practitioners as well as non-technical decision-makers to understand the risks and benefits of incorporating AI into their business.
-
To Future-Proof The Pharma Supply Chain, Digitize
The demand for pharma products is growing — can the industry keep up? Explore three ways digitization can help pharmaceutical companies bridge the widening gap between supply and demand.
-
Establishing Analytical Methods For mRNA-Based Therapies
Here, we provide a detailed description of assays for sequence identification and LNP composition in mRNA-LNP products that support the development of safe and effective mRNA therapies.
-
Fujirebio Increases Study Agility By Streamlining Clinical Operations And Data Management Process
Fujirebio’s fast-growing U.S. Clinical R&D team was preparing for shorter studies with higher complexity. Traditional methods using paper and spreadsheet trackers were no longer adequate to meet quality goals. Learn how the group increased its agility and readiness with LifeSphere Clinical EDC and CTMS tools.
-
Mobile App And Wearable Integration Collects Longitudinal Data
Get an overview of SISCAPA's utilization of eTechnologies, specifically ePRO, and how their partnership with CDS accurately demonstrates the improvement of data collection and analysis in the biotechnology industry.
-
Version2 Gives Customers Direct Access To Information With Secure Documentation Solution
Version2 needed to consolidate all of their clients’ information into one place and automate everyday tasks. Keep reading to learn about how IT Glue helped Version2 create new standards and processes in their business with relationship mapping, integrations, flexible workflows, secure password access, and more.
-
Precision Powder-In-Capsule Micro-Dosing Accelerates Drug Development
Precision powder microdosing makes it possible to quickly get formulations to the clinic, saving time, money, and use of limited APIs.
-
Getting Started With Product Development Strategies
Simplify and streamline new product development for success. Explore the importance of new product innovation, different process methods, and best practices for optimizing your company’s strengths.
-
Modernizing Biopharma Manufacturers To Improve Quality And Safety
Read how proven, scalable digital solutions to modernize pharmaceutical product innovation and development gives enterprises an edge in meeting the manufacturing guidelines for pharmaceuticals while elevating the entire industry.
-
Preparing A New Drug Application (NDA) With A CDISC Conversion
Regulatory submissions are the most critical milestones in clinical research programs. Learn how quality submissions can accelerate time to market, maximize research investments and bring the benefit of new treatments to patients sooner.
-
The Role Of Real World Evidence To Support US FDA And TGA Registrations
Real world evidence (RWE) can contribute valuable information to new medicine registrations, in particular, for diseases that are difficult to assess in randomized clinical trials (RCTs) and for emerging technologies.
-
Rose Research Center Leans On Medrio To Simplify Workflows And Study Design
After experiencing paper processing issues, discover how a company transitioned to digital data collection by using Electronic Data Capture (EDC) and Electronic Patient Reported Outcomes (ePRO) solutions.
NEWS
-
Himax To Present Innovative Solutions For tinyML Opportunities With WiseEye™ AI During CES 202412/20/2023
Himax Technologies, Inc. (Nasdaq: HIMX) (“Himax” or “Company”), a leading supplier and fabless manufacturer of display drivers and other semiconductor products, today announced that during CES 2024 the Company will unveil its new generation WiseEyeTM AI processor, WE2, with a diverse array of new sensor fusion use cases, encompassing image, video and audio.
-
Applications Open For The 2024 SPIE Prism Awards, Which Recognize The Photonics Industry’s Most Innovative Products8/1/2023
Applications are now open for the 2024 SPIE Prism Awards, an event which will celebrate its 16th anniversary on 31 January, during a gala evening at SPIE Photonics West.
-
Aston University Scientists Discover More Accurate Way Of Checking Blood Flow In The Feet Of Type 2 Diabetes Patients2/8/2023
Aston University scientists have discovered a more accurate way of checking the blood flow in the feet of patients with type 2 diabetes.
-
Launching An AI And Photonics Initiative At Duke1/26/2024
AI and photonics have a long history with one another. As the nascent field of machine learning took hold in areas outside of niche programming applications in the 1990s, one of its first major successes was in image recognition.