Web Content Management
PRODUCTS
-
ASEPTICARE offers a versatile, closed aseptic environment, ideal for aseptic and aseptic-toxic operations. It ensures contamination control and improves operational efficiency across various sterile processes.
-
Werum PAS-X as a Service delivers a fully managed, cloud-based MES for pharma and biotech, enabling rapid deployment, scalability, cost savings, compliance, and operational efficiency.
-
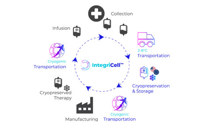
IntegriCell™ cryopreservation services from Cryoport Systems optimize leukapheresis-derived cell therapies by enhancing the safety, quality, and viability of manufacture-ready cryopreserved leukopaks. Our state-of-the-art cryopreservation process, integrated with our end-to-end global temperature-controlled supply chain platform, provides unmatched reliability, efficiency, and scope. Our standardized protocols meet the highest compliance standards, ensuring consistent quality and addressing product stability issues associated with fresh donor-derived cellular material for an optimized approach that enhances manufacturing efficiency and streamlines operations.
-
Designed as a dedicated, short-range logistics service for the life sciences, Cryoshuttle reinforces chain of custody, ensures near real-time visibility, and preserves product integrity from start to finish.
-
Elevate Your Clinical Research Data Collection
WHITE PAPERS AND CASE STUDIES
-
Modernizing Operational Maintenance At Treatment Plants
In 2017, the Harford County Division of Water and Sewer began looking for a modern work management solution to help automate preventive maintenance, coordinate work activities between groups, manage material inventories, and capture important data.
-
Rescuing Data Integrity: A Swift Transition And Quality Transformation
Uncover how this collaboration supported timely progress toward key study milestones, enabling smoother execution and more dependable output across data management and statistical activities.
-
Not Your Typical MES: The Ultimate Guide To Made With MX
Life sciences manufacturing is evolving rapidly. Discover how digital transformation is revolutionizing production processes to enable personalized medicine and improve patient outcomes across the healthcare industry.
-
Mobilizing Compliance: USP 797 And 800 Pharmacy Trailer Fleet For VA Hospitals
VA hospitals across the US are undergoing renovations to bring their facilities into compliance. The challenge was to design and manufacture a fleet of self-sufficient mobile compounding pharmacies.
-
Rescuing A Global Full-Service Phase III Trial For A Late-Stage Oncology Biotech
This case underscores the expertise employed in a comprehensive strategy and set of services that were used to salvage a Phase III clinical trial for ovarian cancer and ensure its timely completion.
-
Revolutionizing European Football With UWB Micro-Location
See how UWB micro-location technology was utilized by European League Football to address pandemic-era challenges, but quickly transformed into a vital tool for player analytics and safety monitoring.
-
Leveraging Single-Use Solutions To Solve Working Cell Bank Challenges
Discover how custom manifolds enhance allogeneic therapy development from formulation to commercialization by improving process control, scalability, and efficiency.
-
Implementing A Disaster Recovery Plan
When facing a malfunction, having an effective disaster recovery plan can make the difference between a swift fix, and losing your samples and time.
-
Overcoming Analytical Bottlenecks In Oligonucleotide Drug Development With Automation
Growing oligonucleotide complexity is straining traditional analytical workflows. See how automated LC‑UV‑MS processing offers a scalable way to boost throughput and reduce manual effort.
-
CDMO Accelerates Contract Manufacturing With Electronic Batch Records
A prominent CDMO sought a specialized software solution to streamline and accelerate its manufacturing processes, enabling the efficient production of complex, customized products in record time.
-
Richter BioLogics Drives Efficiency And Compliance With Unified Quality
Learn how one CDMO tripled production, improved audit readiness, and built customer trust by unifying quality systems and embracing a digital-first approach.
-
A Digital Solution Embeds Quality Into Cellular Therapeutics Production
Discover how Dendreon cut review and release time by nearly 50% while maintaining a 99% right-first-time rate, which boosts collaboration, visibility, and operational efficiency across teams.
NEWS
-
Microbes In Brooklyn Superfund Site Teach Lessons On Fighting Industrial Pollution4/15/2025
NYU Tandon-led research team discovers unprecedented genetic adaptations in Gowanus Canal organisms, revealing a potential new approach for cleaning contaminated waters and recovering valuable resources.
-
Microbot Medical® Announces Emory University Hospital As The First Hospital In The World To Adopt The New LIBERTY® Endovascular Robotic System11/26/2025
Microbot Medical Inc. (Nasdaq: MBOT), developer and distributor of the innovative LIBERTY Endovascular Robotic System, announced that Emory University Hospital, a nationally recognized academic medical center in Atlanta, has become the first hospital to adopt LIBERTY for patient care.
-
Three New Medical Centers Adopt Daxor's Blood Volume Diagnostic Signaling Growing Clinical Acceptance5/6/2025
Daxor Corporation (Nasdaq: DXR), the global leader in blood volume measurement technology, today announces significant expansion of its blood volume analysis (BVA) capabilities in three new hospitals.
-
Rice Rebels: Research Reveals Grain's Brewing Benefits6/30/2025
Christian Schubert and Scott Lafontaine are fighting an old prejudice: that rice doesn't belong in beer. Now they've got the research to upend that ancient bit of brewing snobbery.
-
King Faisal Specialist Hospital And Research Centre Performs The World's First Robotic-Assisted Artificial Heart Pump Implantation1/16/2025
King Faisal Specialist Hospital and Research Centre (KFSHRC) in Riyadh has successfully performed the world’s first robotic-assisted implantation of an artificial heart pump (HeartMate 3) developed by Abbott, a groundbreaking procedure that marks a significant advancement in medical technology and patient care.