Business Process Management
PRODUCTS
-
Liquid-filled two-piece capsules offer advantages like scalability, faster absorption, simpler packaging, and greater product stability. Learn more about their benefits in pharmaceutical development.
-
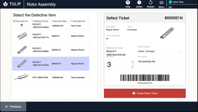
Track and manage defects and non-conformances – such as SOP deviations or missing data – from identification to resolution.
-
For your efficient and error-proof production: Our world-leading Manufacturing Execution System (MES) Suite PAS-X controls, monitors, and documents your processes digitally and in real time throughout the entire manufacturing cycle.
-
As a top Clinical Research Organization, ProPharma brings over 20 years of experience, innovation, personalization, and adaptability to Sponsors’ studies and research.
-
Industry leader in preclinical safety assessments across various therapeutic areas. 30+ years of experience in full range of in vivo non-GLP and GLP studies in both rodent and non-rodent species.
WHITE PAPERS AND CASE STUDIES
-
Digital Transformation And Quality By Design For Enhanced Development
Discover how digital transformation and Quality by Design are revolutionizing pharmaceutical development to boost efficiency, enhance compliance, and accelerate innovation across the drug lifecycle.
-
Re-Engineering A Complex Process For FDA Compliance
Discover the strategies that helped a company navigate a complex and shifting regulatory landscape to rapidly re-engineer a COVID-19 vaccine manufacturing process for FDA compliance.
-
Enabling Simpler, Quieter, And More Precise Wearables Utilizing Disruptive Pump Technology
Disc pumps enable quieter, smaller, and more precise wearable medical devices, improving patient comfort, measurement accuracy, and system reliability for innovative healthcare solutions.
-
End-To-End Workflow Integration For Antibody Development
Modern antibody discovery generates massive, fragmented datasets that slow collaboration and decision‑making. Examine how unified digital workflows streamline R&D and improve data quality.
-
Small Volume ATMP Cryo-Freezing Strategies
Discover best practices for cryopreserving small-volume ATMPs, focusing on sterility, viability, and efficiency with advanced containers and packaging.
-
Enhancing Viral Clearance Prediction And Process Optimization
MockV® technology enables early, in-house viral clearance assessment, helping biopharma developers improve process robustness, reduce costs, and enhance safety before GMP manufacturing begins.
-
Digital Transformation Optimizes Production, Quality, And Compliance
Discover how advanced automation solutions at Fermion's new facility were implemented to enhance production, quality, and compliance in pharmaceutical manufacturing while meeting industry standards.
-
CDMO Uses Robotic Gloveless Isolator For Advanced Therapeutics
CDMOs must innovate production architectures to meet the demands of advanced therapeutics to ensure a reliable supply and compliance. Discover how these approaches advance modern medicine and improve patient outcomes.
-
Quality Assurance In IVT RNA Vaccine Development Using Electrophoresis
Discover advanced solutions for IVT RNA synthesis, focusing on enhancing fidelity in throughput, innovative technologies, and methodologies to optimize your RNA research and development processes.
-
Development Strategies For Adenovirus-Based Gene Therapies
Scaling up viral vector production can be challenging, and ensuring consistent titers and activity requires careful optimization and technical expertise.
-
Overcoming Analytical Bottlenecks In Oligonucleotide Drug Development With Automation
Growing oligonucleotide complexity is straining traditional analytical workflows. See how automated LC‑UV‑MS processing offers a scalable way to boost throughput and reduce manual effort.
-
Pumping And Network Operations Optimized Through Collaborative Digital Twins
Collaborative digital twins streamline utility operations by integrating real-time data into a shared, intuitive environment. This approach enables precise pump optimization, efficient maintenance scheduling, and proactive network simulations to improve water quality and system resilience.
NEWS
-
Siemens Democratizes AI-Driven PCB Design For Small And Medium Electronics Teams5/21/2025
Siemens Digital Industries Software announced today that it is making its AI-enhanced electronic systems design technology more accessible to small and mid-sized businesses (SMB) with PADS Pro Essentials software and Xpedition Standard software.
-
SAP Unveils AI-Powered Innovations For Network-Centric Supply Chain Management5/22/2025
The innovations we unveiled represent a fundamental shift in how businesses can approach their operations in an increasingly complex global landscape.
-
Broadcom Launches VeloSky To Deliver Network Convergence, Transform Connectivity3/4/2025
Mobile World Congress 2025—Broadcom Inc. (NASDAQ: AVGO) today introduced VeloSky, a converged networking solution that enables Communications Service Providers (CSPs) to offer integrated fiber, cellular, and satellite connectivity through a single appliance.
-
FieldPulse Announces Integration With The Granite Group, Enhancing Efficiency For Contractors And Trade Professionals3/18/2025
FieldPulse has launched a new integration with The Granite Group.
-
Samsara Launches Its Most Compact Asset Tag Yet2/26/2026
Samsara Inc., the pioneer of the Connected Operations Platform, today announced its latest-generation Asset Tag and all-new Asset Tag XS, designed to help operations and fleet equipment managers track and recover high-value assets of all sizes.