Compliance
PRODUCTS
-
PHCbi brand's 10.4 cu.ft. (294 L) humidified plant growth chamber is designed to helps ensure highly accurate, reproducible chamber conditions. It allows for precise control of temperature, humidity, and lighting - making it ideal for drosophila breeding, diurnal growth studies, plant cell culturing and more. Robust cabinet construction with corrosion-resistant stainless-steel surfaces simplify cleaning and eliminate rust due to high humidity. It uses SNAP compliant, low flammability HFO refrigerants to assure low environmental impact.
-
Discover how a collaborative approach to medical writing ensures the highest standards of excellence and precision in every document produced.
-
Conduct secure video based visits, manage study schedules, and keep participants connected through a single unified platform.
-
Whenever possible, Mueller tailors product certification to match specific job requirements, including part numbers, job identification, and funding agency requirements. We have developed this process working closely with the Environmental Protection Agency (EPA) who was very early in clarifying definitions and requirements for Iron & Steel Products, Construction Materials, and other Manufactured Products.
-
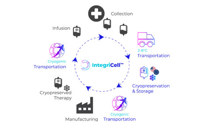
IntegriCell™ cryopreservation services from Cryoport Systems optimize leukapheresis-derived cell therapies by enhancing the safety, quality, and viability of manufacture-ready cryopreserved leukopaks. Our state-of-the-art cryopreservation process, integrated with our end-to-end global temperature-controlled supply chain platform, provides unmatched reliability, efficiency, and scope. Our standardized protocols meet the highest compliance standards, ensuring consistent quality and addressing product stability issues associated with fresh donor-derived cellular material for an optimized approach that enhances manufacturing efficiency and streamlines operations.
WHITE PAPERS AND CASE STUDIES
-
Q&A: Payment Processing With Precidia Technologies Precidia has issued several press releases, through late 2008 and early 2009 regarding the TransNet solution. What is your strategy with this product? In introducing TransNet, we are offering the market an alternative to the established integrated payment model. Our solution addresses some of the limitations of the...
-
An Equitable Way To Meet On-Site Business Needs
Tackling high turnover and employee equity challenges is a priority. Learn how an on-site insourcing solution can provide a stable and motivated team, ensuring project success.
-
Maximize Output From Full-Time Employees
Learn about project-based insourcing solutions designed to give clients laboratory services support with flexible timeframes, and eliminate worries about co-employment and other regulatory concerns.
-
Adopting Label Change Best Practice To Promote Clarity And Compliance
Globalized clinical trials are quickly becoming a pre-requisite for success. Read Almac’s latest case study to learn how one sponsor was able to extend its trial into new countries without risking labelling non-compliance, which enabled them to continue on their mission to improve the health outcomes for millions of patients.
-
Fire And Life Safety And Security Industry
New government regulations and the need to update outdated or non-compliant technology are requiring building owners and facilities managers to think about upgrading their fire alarm, security, and fire suppression systems with the latest technology available.
-
Cabrini Health Experiences Improved Operational Efficiency And Other Significant Benefits With triCerat
Cabrini Health is a not-for-profit Catholic healthcare service with 3,800 employees and over 700 beds comprising of two acute care hospitals, a palliative care service, rehabilitation hospital, residential care facility, and pharmacy department.
-
Focus On Your Core Business Strategy
Learn how a large manufacturing client helped to focus on their core business with a solution that transitioned approximately 60% of the workforce and capitalized on the initial training investment.
-
Comparative Analysis For Non-IND Sites
A cancer research organization that facilitates multi-national clinical trials was in the planning and coordination of a Phase III drug trial and wanted to expand the trial to include German sites. Conducted under an IND, the trial was going according to plan until German regulations changed.
-
Preparing For Post-Market Clinical Follow-Up Under EU MDR
Gain a better understanding of the primary challenges faced by the life sciences industry's commercial model in response to the updated EU MDR guidelines.
-
Allos Faced A Diverse Set Of Business Challenges Allos Therapeutics, Inc. is a biopharmaceutical company focused on developing and commercializing innovative small molecule therapeutics for the treatment of cancer. Allos has 75 employees in offices in Colorado and New Jersey. Especially with distributed employees, a paper-based system was not meeting the company’s needs for information sharing and collaboration. By KnowledgeTree, Inc.
-
How Social Technologies Are Creating A New Paradigm For Addressing Business Objectives
This case study investigates how Within3 supported a culture of innovation by introducing three pharmaceutical companies to secure, regulatory-compliant virtual advisory boards. By Within3
-
24/7 Cell & Gene Control Tower Provides Agile Solution For Critical Shipments
Get an overview of a successful re-route of six critical shipments to the UK and Spain from Amsterdam used to deliver CAR-T drug products to treat non-Hodgkin’s lymphoma cancer patients.
NEWS
-
Curia Invests $4M To Enhance Sterile API Manufacturing10/27/2025
Curia, a leading global research, development and manufacturing organization, today announced the completion of a $4M investment to upgrade its two API aseptic suites in Valladolid, Spain.
-
AppTec360's Advanced Mobile Content Management Solutions Reinforce Enterprise Security3/11/2025
Data security remains a critical concern for organizations as the risk of breaches and unauthorized information sharing continues to rise. Addressing these challenges, AppTec360 introduces ContentBox, a Mobile Content Management (MCM) solution designed to enhance security while enabling seamless file synchronization and controlled data access.
-
KETOS Launches In-House Environmental Lab Testing For Water Quality4/23/2025
KETOS, a leader in automated water testing solutions, today announced KETOS Environmental Lab Platform, its new in-house Environmental Lab Testing Services for residential and commercial clients.
-
airSlate SignNow And Paperwise Partner To Redefine eSignature And Workflow Automation3/10/2025
airSlate, a global leader in electronic signature and document workflow automation, is excited to announce a new integration and reseller partnership with Paperwise, a premier provider of process automation and document management solutions.
-
Rise Expands Employer Of Record Services To Revolutionize Global Hiring And Payroll Compliance4/3/2025
Rise, the pioneering Web3-enabled payroll and compliance platform, has announced the expansion of its Employer of Record (EOR) services, enabling businesses to seamlessly hire full-time employees across borders without the need to establish local entities.